Introduction
Gel permeation chromatography (GPC) or, equivalently, size-exclusion chromatography (SEC) is a widely used technique to characterize a wide variety of macromolecules, including, but not limited to commodity or bulk manufactured polymers. This technique can be used to measure the molecular weight moments, molecular weight distribution, the intrinsic viscosity, and/or hydrodynamic size of these macromolecules. Figure 1 shows a complete setup for a GPC system.
Figure1: Malvern Viscotek TDA Triple Detection GPC System
While conditions that are both effective and practical have been determined for many of these commodity polymers (i.e., THF for polystyrene, chlorinated benzenes for polyolefins), others need further optimization. This application note describes one such polymer, nylon, where changes in operating conditions can lead to improvements in both cost and health and safety.
GPC Analysis of Nylon
Traditional Analysis of Nylon Typical GPC analysis of nylons has involved the use of phenolic based solvents (o-chlorophenol, m-cresol, phenol blends) or hexafluoroisopropanol (HFIP) for the dissolution solvent and/or mobile phase. Of these solvents, HFIP provides significant benefits over the phenolic solvents with lower quantifiable health and safety hazards (i.e., higher LD50 limits) and substantially higher signal-to-noise ratios in both refractive index and light scattering detectors due to a higher dn/dc. The main drawback to HFIP is the potentially prohibitive cost which can range from $2 to $4 (or more) per mL. Formic Acid Analysis of Nylon Formic acid has historically been used to measure the solution viscosity of nylon. From the standpoint of the metrics mentioned previously, formic acid is equivalent or better than HFIP with respect to health and safety. The LD50 limit is slightly higher with formic acid, and the significantly lower vapor pressure of formic acid inherently leads to a lower potential for inhalation exposure. On the issue of price, formic acid costs significantly less than HFIP, in the range of $0.15 to $0.30 per mL. Some drawbacks to using formic acid as a mobile phase are that it does not dissolve all forms of nylon, specifically nylon-11 and nylon-12. Also, formic acid can be extremely caustic to many of the traditional components of liquid chromatography systems. The Viscotek TDA Triple Detection GPC System, shown in Figure 1 and used for this study, has specific, proprietary design components that allow for the use of formic acid as a mobile phase. Finally, dissolved nylon samples will have a decreased signal-to-noise ratio compared to HFIP due to a lower dn/dc of these samples in formic acid. The following section illustrates whether or not that decreased signal-to-noise ratios would prevent adequate GPC analysis of nylons in formic acid. GPC Results A series of four nylon-6 samples were prepared twice for GPC analysis, once for analysis in a mobile phase of HFIP with 0.05M potassium trifluoroacetate and once for analysis in a mobile phase of 88% formic acid. Figure 2 shows a triple detector chromatogram of one of the samples in HFIP, and Figure 3 shows a triple detector chromatogram of the same sample in formic acid. The following chromatograms show the refractive index signal in red, the viscometer signal in blue, and the right angle light scattering (RALS) signal in green. The calculated molecular weight at each retention volume is also shown in black to visualize the sample’s molecular weight distribution.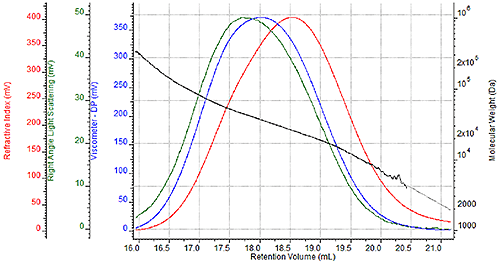
Figure 2. Triple detector chromatogram of nylon-6 sample in HFIP
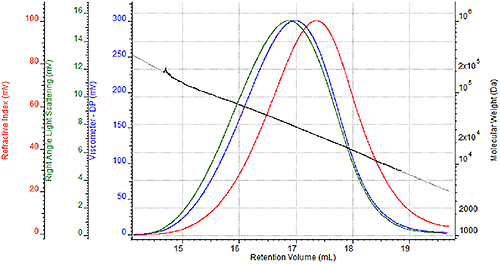
Figure 3. Triple detector chromatogram of nylon-6 sample in 88% formic acid
While the chromatograms and associated molecular weight distributions appear similar with the two different mobile phases, a more detailed numerical comparison is needed to accurately assess the efficacy of formic acid as a mobile phase.
Summary of Results
Table 1 below compares the molecular data obtained for the four nylon-6 samples using HFIP as the mobile phase and using 88% formic acid (FA) as the mobile phase. Presented values include the moments of the molecular weight distribution (Mn, Mw, and Mz) and intrinsic viscosity ([η]). Data presented are the average of three injections.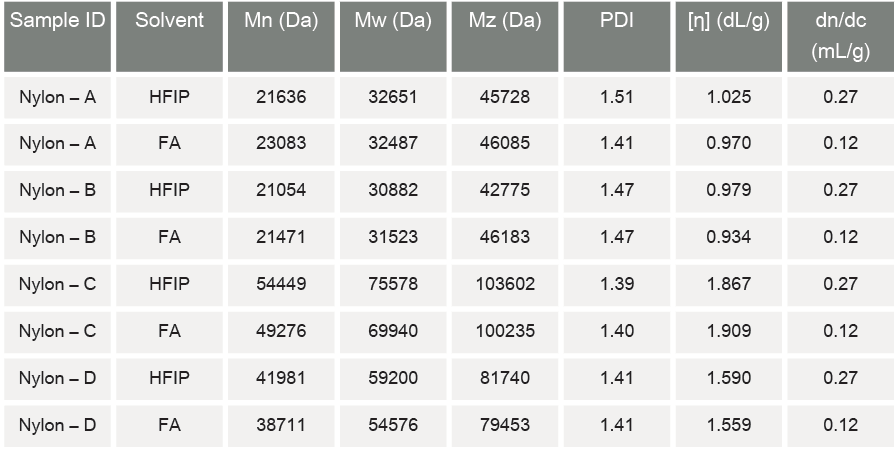
Table 1. Molecular data derived for four nylon-6 samples in HFIP and 88% formic acid
Table 1 shows the calculated data for the different nylon samples in each mobile phase. The molecular weight distribution is nearly identical for all samples (< 10% difference). The [η] are also correlates nicely between the two different mobile phase. In this instance, the correlation is near unity, but this is more of a coincidence. Different mobile phases could swell the polymer chains to different degrees which would lead to different [η] for the same sample. For a cost comparison, running these samples in HFIP cost more than $1600 while running these same samples in 88% formic acid cost less than $125. In fact, the total cost of running all the samples in formic acid (~$125) is less than the cost of running a single injection of one of the samples in HFIP (~$140).
Conclusions
For the GPC analysis of nylon-6, nylon-6,6, or potentially other soluble polyamides, formic acid provides an effective, inexpensive, and safer alternative to mobile phases traditionally used for these polymers. The Viscotek TDA Triple Detection GPC System provides outstanding signal-to-noise ratios for the robust and precise analysis of nylons in formic acid. In addition, the Malvern GPC system has been specifically designed to reliably operate under the potentially corrosive conditions posed by formic acid and other solvents.Malvern provides the materials and biophysical characterization technology and expertise that enables scientists and engineers to investigate, understand and control the properties of dispersed systems. These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries. Used at all stages of research, development and manufacturing, Malvern’s instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency. Our products reflect Malvern’s drive to exploit the latest technological innovations. They are used by both industry and academia, in sectors ranging from pharmaceuticals and biopharmaceuticals to bulk chemicals, cement, plastics and polymers, energy and the environment. Malvern systems are used to measure particle size, particle shape, zeta potential, protein charge, molecular weight, mass, size and conformation, rheological properties and for chemical identification, advancing the understanding of dispersed systems across many different industries and applications. www.malvern.com Material relationships http://www.malvern.com/en/






