Volatile organic compounds are used in pharmaceutical manufacturing during the production of drug substances, pharmaceutical additives, and drug products. Known also as residual solvents, they account for 50-90 percent of mass in typical pharmaceutical operations and represent most of the process toxicity. Testing for the presence of these solvents, therefore, is critical for patient safety. Testing commonly follows United States Pharmacopeia (USP) Method <467> guidelines, which suggest a gas chromatography (GC) runtime of 60 min. In addition, it is recommended that Class 1 and 2 solvents be identified and quantitated by complementary methods utilizing different stationary phase selectivity when solvents are found to meet or exceed the permitted daily exposure (PDE) concentration limits. In this unique application, a gas chromatography–vacuum ultraviolet (GC-VUV; VUV Analytics VGA-100) method was developed to significantly reduce the analytical bottleneck involved in the testing of residual solvents. Experiments were undertaken to demonstrate the chromatographic capabilities of GC-VUV when applied to residual solvent analysis using model pharmaceutical matrices such as generic throat spray. Samples were prepared by mixing 2-mL of water with 25-100 mg/mL of pharmaceutical product and spiking Class 2 Solvent Mix A and B (Restek) to meet the desired concentration limits. The total GC runtime was set to eight minutes and used throughout the GC-VUV residual solvent application.
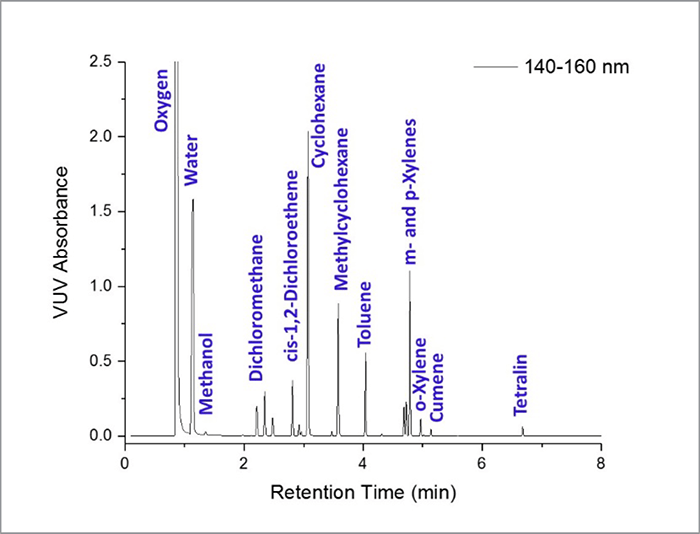
This method for residual solvent analysis was shown to offer a few key advantages over USP Method 467 experimental conditions. First, VUV absorbance spectra provided authoritative solvent identification as well as compound quantitation. Second, chromatographic compression was possible without any loss of data quality. Runtimes of 8 minutes or less – a 5× or greater reduction – were shown to be sufficient for the analysis of Class 1 and 2 residual solvents. Third, different residual solvent classes could be analyzed in a single run. The VUV detector delivered a linear, highly reproducible response at concentrations ranging from 2× to 0.1× the Class 1 and 2 PDE detection, and the ability to deconvolve analyte co-elution. In summary: the analysis of residual solvents by GC-VUV delivers a single method for the identification and quantitation of toxic compounds in pharmaceutical products.

